Epigenetic changes are associated with various inflammatory diseases and are influenced by environmental factors. Recent data support an association between titanium dissolution products and peri-implantitis.
Other relevant polymorphisms adversely impacting on bone health can increase the risk of peri-implantitis by affecting the rate of bone turnover, the bone mineral density and the formation of the collagen based bone matrix.
Implant surface characteristics, as well as physical and mechanical properties, are responsible for the positive interaction between the dental implant, the bone and the surrounding soft tissues.
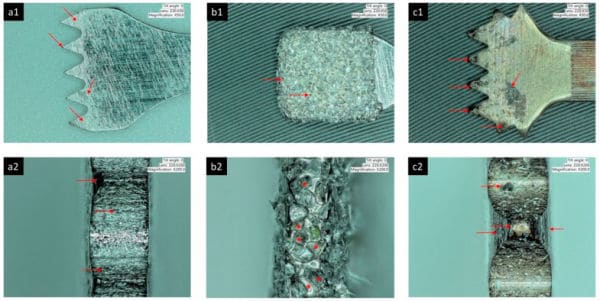
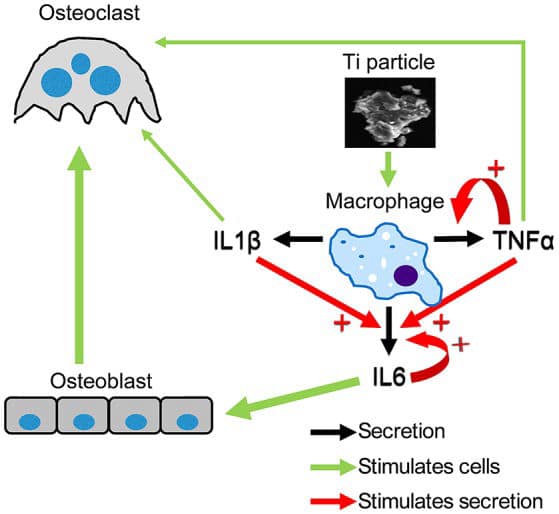
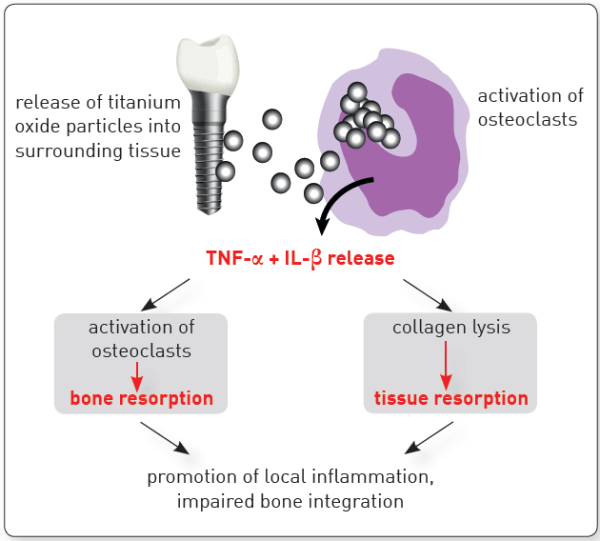
Degenerative changes were reported in macrophages and neutrophils that phagocytosed titanium microparticles, and mutations occurred in human cells cultured in medium containing titanium-based nanoparticles.
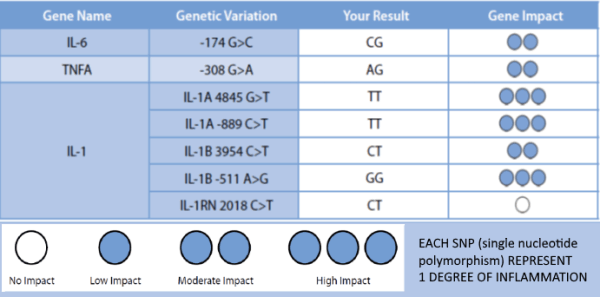
A variety of studies have shown the relationship between peri-implantitis and functionally relevant polymorphisms in the genes of cytokines IL-1A, IL-1B, IL-RN and TNFA.
Using genetic testing allows for the allocation of a certain degree of inflammation to the detected combination of alleles.
Patients with degree 3-4 are considered high responders and are thus risk patients for titanium associated inflammatory processes/ loss of implant.
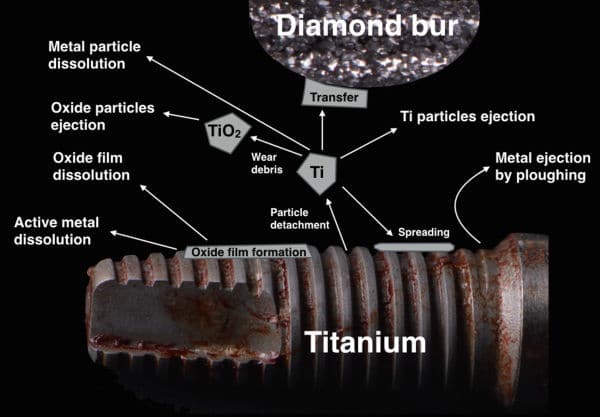
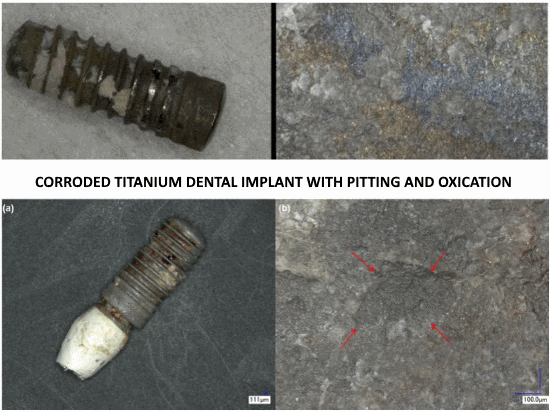
Particulate metal was identified in peri-implantitis and control biopsies, but element analyses could confirm only the presence of Ti in peri-implantitis tissue.
This study evaluates diagnostic markers to predict titanium implant failure. Retrospectively, implant outcome was scored in 109 subjects who had undergone titanium implant surgery, IL1A -889 C/T (rs1800587), IL1B +3954 C/T (rs1143634), IL1RN +2018 T/C (rs419598) and TNFA -308 G/A (rs1800629) genotyping, in vitro IL-1β/TNF-α release assays and lymphocyte transformation tests during treatment.
Implants with peri-implantitis harbored significantly higher mean levels of titanium (0.85 ± 2.47) versus healthy implants (0.07 ± 0.19) after adjusting for amount of plaque collected per site (P = 0.033).
Here we show that a strong inflammatory response occurs; however, very few of the titanium particles are phagocytosed by the macrophages. We then measured a dramatic Ti particle-induced stimulation of IL1β, IL6, and TNFα secretion by these macrophages using multiplex immunoassay. The particle-induced expression profile, examined by FACS, also indicated an M1 macrophage polarization.
Metal ions in concentrations representing the platform-matched groups led to a reduction in cell viability (P<0.01) up to 7days of exposure. Stimulated cells showed higher rates of early apoptosis (P<0.01) compared to non-treated cells.