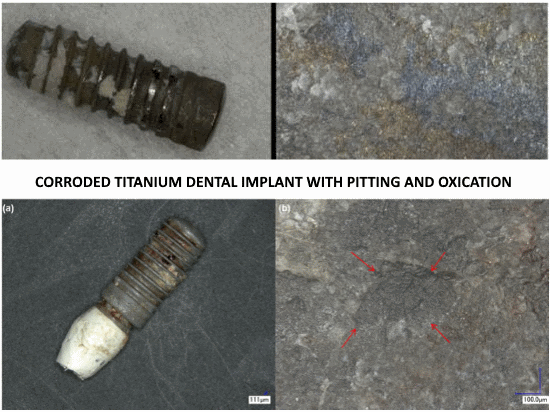
Despite high biocompatibility of titanium and its alloys, this metal causes various side effects in the human body. It is believed that titanium biomaterials may induce an innate/adaptive immune response. However, still little is known about changes caused by titanium mandible implants, particularly with regard to bone healing.
Tag Archives: titanium
Titanium implants are routinely used for bone fractures as well as dental work. It has recently been shown that titanium-based implants both corrode and degrade, generating metallic debris. There is some concern over the increased concentrations of circulating metal-degradation products derived from these implants, and their potential harmful biological effects over a period of time, including hepatic injury and renal lesions.
This study indicates that titanium is unsuitable as a biomaterial in devices which are in direct contact with blood for a prolonged period.
Recent data support the implication of accelerated titanium dissolution products in peri-implantitis. It is unknown whether these dissolution products have an effect on the peri-implant microbiome, the target of existing peri-implantitis therapies.
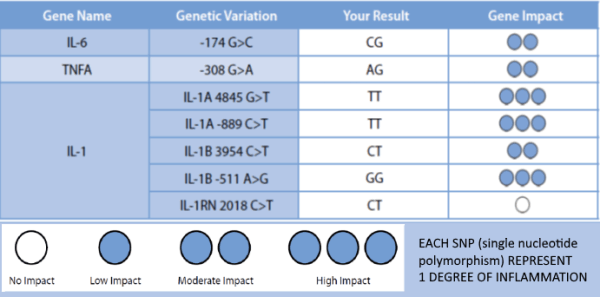
A variety of studies have shown the relationship between peri-implantitis and functionally relevant polymorphisms in the genes of cytokines IL-1A, IL-1B, IL-RN and TNFA.
Using genetic testing allows for the allocation of a certain degree of inflammation to the detected combination of alleles.
Patients with degree 3-4 are considered high responders and are thus risk patients for titanium associated inflammatory processes/ loss of implant.
Particulate metal was identified in peri-implantitis and control biopsies, but element analyses could confirm only the presence of Ti in peri-implantitis tissue.
This study evaluates diagnostic markers to predict titanium implant failure. Retrospectively, implant outcome was scored in 109 subjects who had undergone titanium implant surgery, IL1A -889 C/T (rs1800587), IL1B +3954 C/T (rs1143634), IL1RN +2018 T/C (rs419598) and TNFA -308 G/A (rs1800629) genotyping, in vitro IL-1β/TNF-α release assays and lymphocyte transformation tests during treatment.